It’s time for the last experiment of Chapter 1
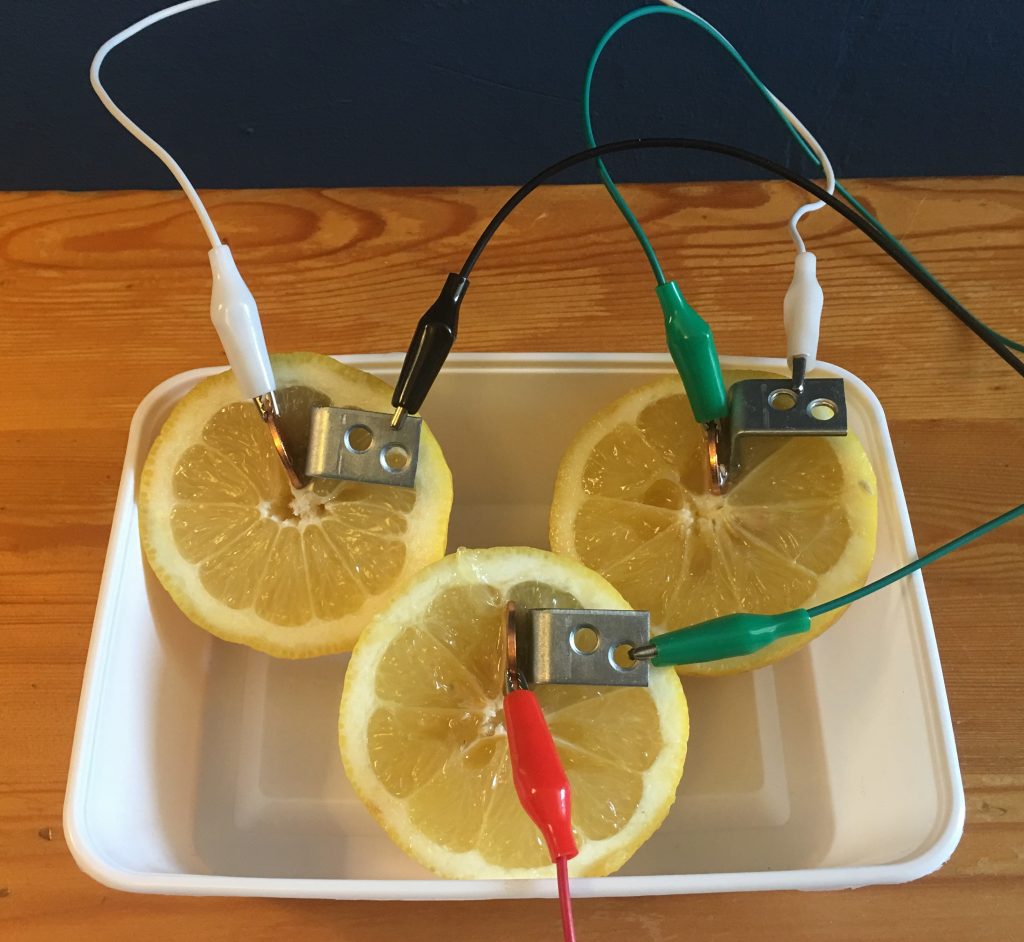
I found some really cheap L-shaped 1in. Zinc-plated steel brackets at my local hardware store.
I was unable to light up the LED with this setup
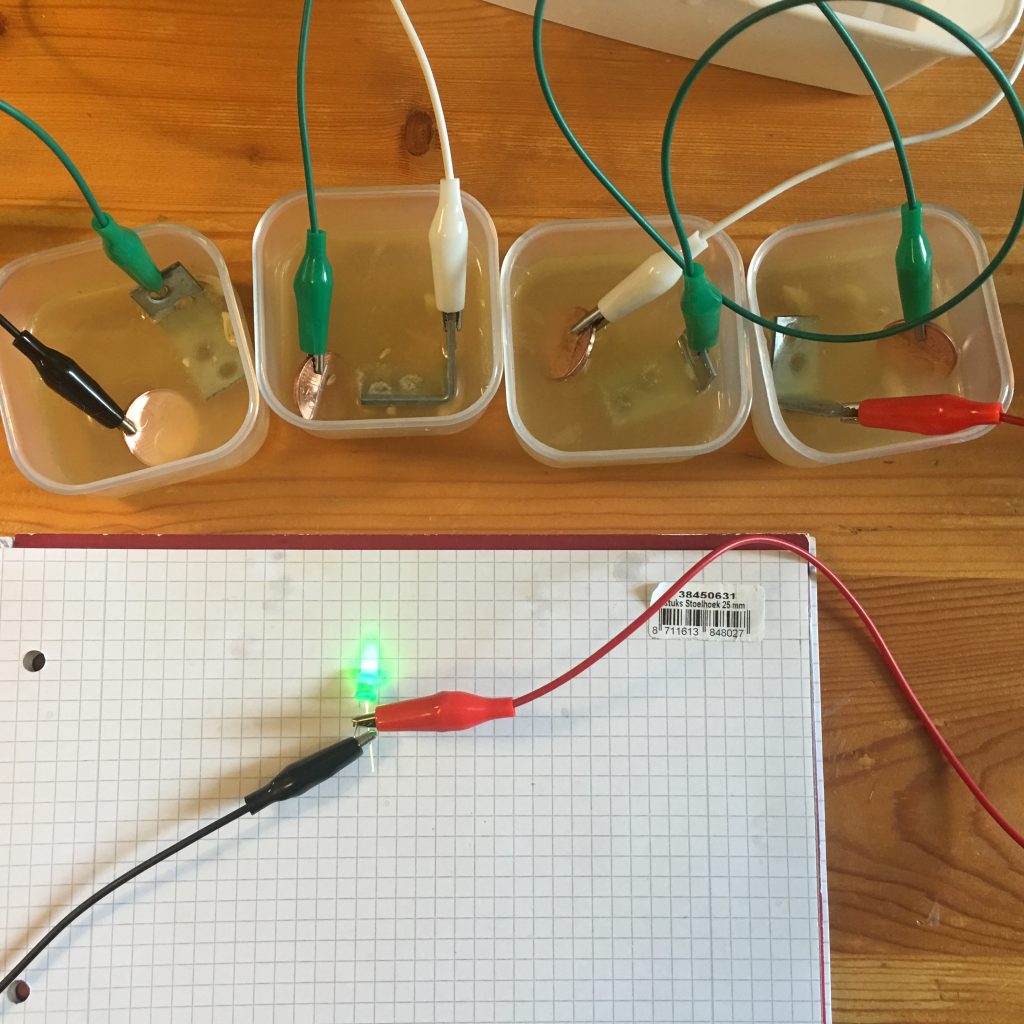
Luckily I found some small plastic containers at a dollar store and was able to imitate Mr. Platt’s second setup. This setup delivers more voltage since we’re connecting more pairs of electrodes (brackets and pennies) in series.
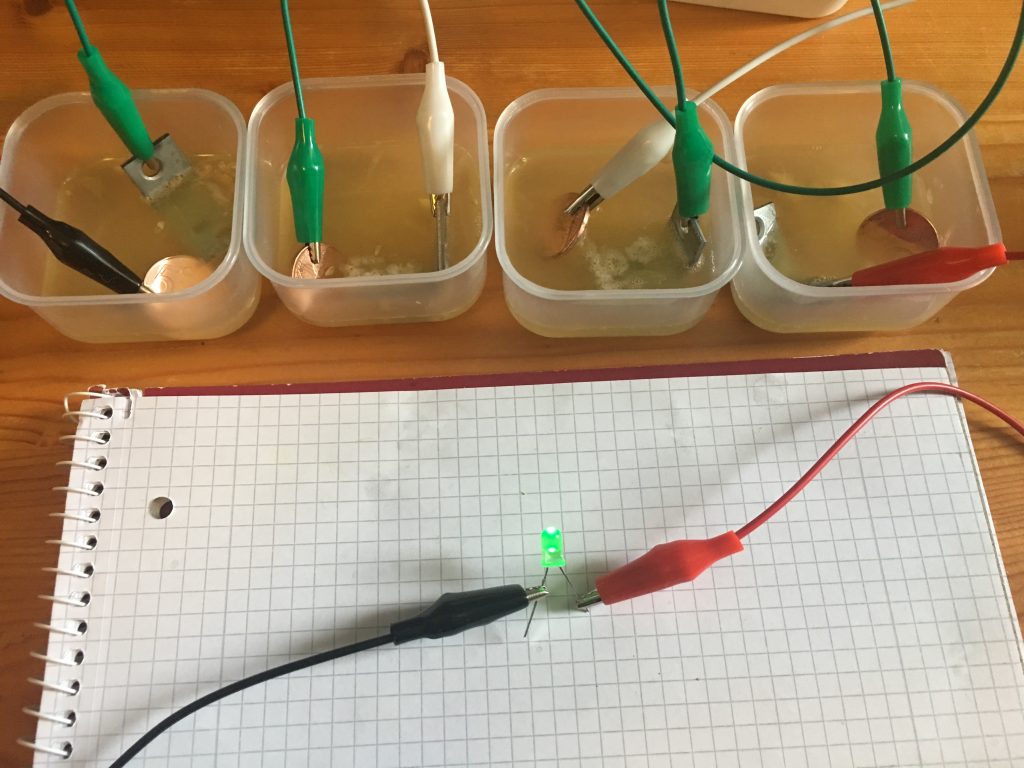
Shine bright like a LED!
The highest voltage reading with this setup was 3.8V, which is higher than I was expecting. So be sure to measure the voltage of your circuit before connecting the LED to it. If the voltage measured is higher than the Max Forward voltage of your LED (check its datasheet), you could end up with another burnt LED like in experiment 4, or would you?
Turns out that while it is a bad idea to apply more than the recommended forward voltage to an LED, a lemon battery may be unable to sustain a voltage when it has a load applied to it (such as an LED). It would then be interesting to measure the voltage of the lemon battery with and without the LED attached (the voltage drops significantly when you add the LED).
The current is also important, the internal resistance of a battery limits current, and current is the main factor burning out an LED.
For these reasons, it’s safe to say you won’t end up with a burnt LED when you connect it to our lemon battery!
The Voltage eventually settled at 2.1V
Be sure to carefully read through the theory section of this experiment. A basic understanding of the relationship between voltage, current, and resistance will greatly help your understanding of electricity and how and why circuits are formed.
To keep your test leads in good shape, clean them with soap and water then quickly dry them to avoid rusting.
Time for Chapter 2!